Hey Friends!
Ready to have fun with kids?
Welcome to the first episode in STEM KIDS category.
I hope to share some fun filled “experiments” regularly that you can do with kids using home based ingredients. I will also outline the basic science principles underlying these experiments as well, and introduce some scientific terminologies. You can discuss these with your kids as appropriate for their age and level of science education.
So let’s get started!
Experiment: Underwater Fireworks (Age 2-8)
Things Needed:
Food Colorings (at least 3, the more the merrier),
Test tube (with test tube stand) OR Transparent water glass/jar 1
Measuring cup
Stir stick OR spoon/fork
Some water AND some vegetable oi
Step 1: Pour 40ml water to test tube and add 10 ml vegetable oil
(these measurements would change if you use a drinking glass, you can decide the volume based on that. However ensure to leave some space at the top)
Question 1: What do you observe? Why?
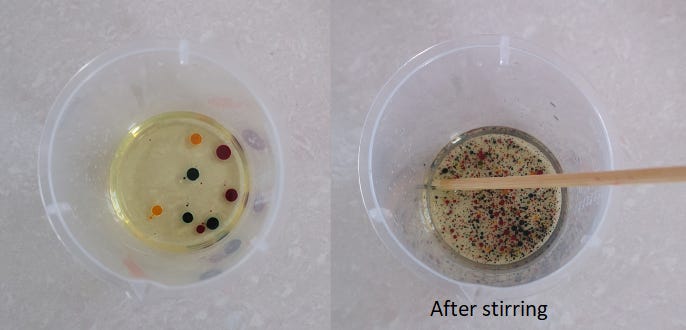
Step 2: Add 5 ml vegetable oil to the measuring cup. Add 3 drops of each different coloring and stir
Question 2: What happens to the coloring drops? Why?
Step 3: Pour the oil mixed with coloring into the test tube
Enjoy watching rainbow fireworks appear under water!
Question 3: Why does the firework happen in water and not initially in oil?
Question 4: Why does it take some time for the food colour to fall through the oil?
Science behind the Experiment:
Answer for Question 1:
Observation:
Oil layer floats on water.
It does not mix with water.
Reason:
Water is a polar solvent while oil is a non-polar solvent. There’s a famous quote in Chemistry: “Like dissolves Like”. i.e. Polar substance dissolve in polar solvents and vice versa. Hence oil does not dissolve in water.
Density of oil is less than that of water thus it floats on water.
Answer for Question 2:
Observation:
The food colouring will turn into little spheres - it does not mix with the oil. After few seconds, it falls to the bottom of the oil. Even after you mix it up, it breaks down into lots of smaller spheres like below:
The Reason: Food colouring is essentially pigments dissolved in water; hence it is water based and does not dissolve in oil. Also, its density is higher than that of oil making it sink to the bottom.
Answer for Question 3:
Same explanation and principle as above. For the “fireworks” to form, the pigments should contact water and dissolve (both are polar). Since oil is non polar, it is not facilitated.
Answer for Question 4:
The main reason is: Surface Tension. As mentioned before, since they don’t mix, these coloring “spheres” have to navigate through this oil “surrounding”. Eventually, the surface tension breaks, forms gaps and the coloring “spheres” are able to pass through.
Explaining the Science Terms to kids:
Density: Density refers to how much mass there is in a particular space/volume.
Take 2 similar boxes (A and B). Fill A with 10 balls and B with 6. Even though the size “looks” the same, A has more density than B.
Similarly, take 2 boxes of differnt sizes (e.g. C is larger than D). Fill each of them with 5 balls. Even though C is larger, it has less density than D.
Polar Solvent: Polar solvents have a “positive” and a “negative” charge at different places in their structures and Non polar substances do not.
Surface Tension: All the molecules at the surface are pulled together to form a tight skin like barrier.
Leave a comment below and tell me how you enjoyed doing this activity with the kids! Children are naturally curious. What questions did they ask you?
I hope to share the importance of STEM education for kids along with more fun experiments in future issues of the newsletter. So stay tuned :)
See you next week. Much Love,
Rina Faisal